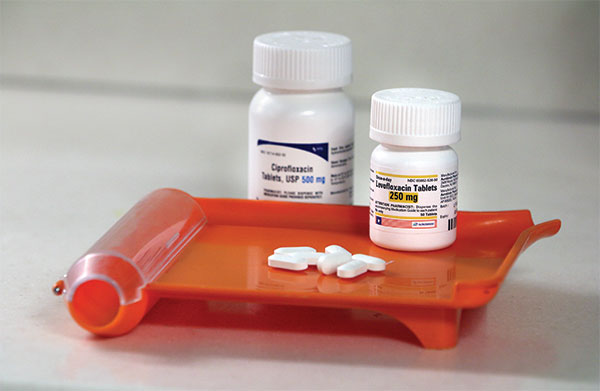
Researchers say some powerful antibiotics pose risks that should be acknowledged.
Antibiotics Fluoroquinolone Drugs
Rx warning: Possible side effects from some antibiotics
Ryne Danielson | MUSC News Center | April 7, 2015
http://academicdepartments.musc.edu/pr/newscenter/2015/antibiotic-warning.Html#.Vhgn7flVikr
________________________________________
Sarah Pack

Researchers say some powerful antibiotics pose risks that should be
acknowledged.
________________________________________
When Raja Fayad, M.D., was murdered on the campus of the University of South Carolina this past February, he was working on something big, something Charles Bennett, M.D., Ph.D., hopes will be his legacy.
Bennett is endowed chair in medication safety and efficacy at the South Carolina College of Pharmacy, a partnership between USC and MUSC. Bennett also runs one of the largest and most successful pharmaceutical watchdog groups in the country, the Southern Network on Adverse Reactions—SONAR.
In June, SONAR filed a citizen petition with the Food and Drug Administration regarding fluoroquinolones, a class of drug that includes the powerful antibiotics Levaquin and Cipro. Bennett wants “possible mitochondrial toxicity” and “serious psychiatric events” added to the drugs’ black box labels — the most prominent warning label required by the FDA.
SONAR has gathered testimony from thousands of patients all across the country, patients who experienced muscle weakness, chronic fatigue, cardiomyopathy, hearing loss, developmental disorders, severe depression or nerve damage after taking Levaquin and related drugs.

Dr. Raja Fayad was waiting for publication of his paper when he was
killed.
Bennett has also linked neurodegenerative diseases like Parkinson’s, Alzheimer’s and amyotrophic lateral sclerosis (ALS) to the drugs and the mitochondrial toxicity he believes they cause in some patients.
The problem: most patients – even most doctors – don't know these side effects exist. On top of that, the most common use of the drugs is off-label, that is, for uses other than those explicitly approved by the FDA.
Bennett gave an example: "A patient goes to see their doctor on a Friday afternoon because they don't feel well. What we should do is watch that patient closely, see if they really have an infection; they probably don't. The patient hates that. On the other hand, give them a prescription for Levaquin and they think, 'my doctor's taking good care of me.'"
The drugs are often prescribed, Bennett said, to placate patients – and doctors themselves.
“The antibiotics are viewed as harmless and effective,” he said. “So, why not?”
But, he stressed, the drugs are only appropriate when used as directed.
Companies are not allowed to market their drugs off-label, but doctors are allowed to prescribe drugs, including fluoroquinolones, for off-label use. Seventy-five percent of all prescriptions are written off-label, Bennett said. This presents problems when doctors are not fully aware of the possible side effects associated with the drugs they are prescribing, which is why Bennett believes getting a black box warning is so important.

Dr. Charles Bennett runs one of the most successful pharmaceutical watchdog
groups in the country.
Bennett is clear: he doesn't want the drugs banned. Levaquin and Cipro are important drugs, especially in clinical ontology where they are used to treat secondary infections in patients undergoing chemotherapy.
"Every drug has side effects," Bennett said. "If the drug has a good risk-benefit profile, then it should be on the market.”
Side effects can be prevented, he explained. They can be treated. For mitochondrial toxicity syndrome, he believes, the risk can be mitigated with a simple genetic test. But, first the risks have to be acknowledged.
SONAR is the only pharmaceutical watchdog group that focuses specifically on drugs used in the treatment of cancer.
“We have a niche that nobody else in the country can be in,” Bennett said. “The science is too hard. You have to understand oncology.”
Bennett is an oncologist. It is this clinical background that sets him apart in his field. Being a clinician, he believes, is very different from being a statistician, which is what most other watchdogs are. Rather than combing large databases for adverse drug reactions, Bennett goes to the patients themselves.
SONAR is a fitting acronym. “SONAR listens to people who have been harmed by an adverse drug reaction," Bennett said. "We give them a voice.”
He continued, “If a patient takes a drug and experiences a terrible side effect, what could they do? What could they do? They’d be upset, I know that. But, what could an ordinary citizen do?
“One option might be to call their doctor. What would the doctor say? ‘That’s a terrible thing that’s happened to you, I’ll put it in your notes.’ The patient wouldn’t feel very good about that.
“So, they’d say, ‘Let me call the FDA,’ and they’ll call up some number in Washington. They’re put through a series of push-ones and push-twos, until they get to a person that says, ‘That’s a terrible thing that’s happened to you, could you please send us a two-page report?’
“The FDA gets more than 250,000 such reports every year. The patient would be filed away and never even get a thank-you note.
“Then the patient might call the drug company. One can imagine how that would go. The drug company will have a drug safety representative who is required to file a report with the FDA within 15 days. They’ll say, ‘That’s a terrible thing,’ and file their report. But, at the end of the day, the patient is back at square one.
“A final option,” Bennett said, “is SONAR.”
Bennett’s group has personally collected hundreds of patients’ stories from across the country, in addition to the more than 130,000 adverse event reports filed with the FDA.
“Levaquin, Ciprol — these are billion-dollar drugs,” Bennett said. “But the FDA has the authority and responsibility to ensure they’re labeled correctly.”
Before Fayad was killed, he was engaged in documenting these side effects in a clinical setting, adding empirical evidence to a surfeit of anecdotal accounts. Fayad’s paper, submitted last summer, has been under review for eight months. This is an unusually long time, Bennett said, saying it amounted to a “pocket veto.” Since Fayad is no longer able to fight for his work, Bennett is stepping in.
“I wrote a letter to the editor and said I need this paper adjudicated,” Bennett explained. “I got a commitment that that would happen.”
Bennett said the pushback from drug companies has been "unbelievable."
The companies he has taken on have a market capitalization in excess of $700 trillion; their products are worth hundreds of billions of dollars.
“You can’t do what I do and think the drug companies don’t notice you,” he said. “Wyeth, Schering-Plough, Eli Lilly, Novartis, Roche, Genentech—you can’t find a major drug company that SONAR hasn’t found an issue with.”
Bennett said many things can happen when one finds themselves on the wrong end of a powerful corporation: “Sometimes you find that you’re not invited to conferences you used to be invited to. You’re kicked off committees. Sometimes they send private investigators after you.”
“They’ve looked at everything I’ve ever done in my life,” Bennett said. “At the end of the day, most people would not do my work. It’s far too dangerous. I’ve paid a tremendous personal cost.”
In 2008, Bennett was investigated for misappropriating NIH funds, a charge he later settled with the government without fault.
“People wanted to know, where do you get your money, how do you spend your money, can you account for it all? So, the federal government decided to audit everything we’d done for the last 15 years.”
Northwestern University, in Chicago, where Bennett used to work, was fined $3 million for misadministering grants. The employee in charge of those grants went to jail.
“That’s an aggressive action,” Bennett said. “And the employee that made the allegations against me was a temporary employee that had worked there eight weeks. There’s no way a temporary employee can, in eight weeks, identify misadministration of grant funding that allegedly took place over a decade. It was a concerted effort to shut my program down. But, the program is not shut down. We will be heard.”
On top of all that, Bennett said, the pushback comes not only from the drug companies, but from the FDA itself. After filing a Freedom of Information Act request with the FDA, Bennett discovered the agency’s own advisory council had investigated reports related to fluoroquinolones and found the same side effects Bennett found. The FDA advisory council reported that mitochondrial toxicity effects were not appropriately addressed on the drugs’ package inserts, but no actions have yet been taken as a result of that report.
“The FDA is not in the business of pushing back very hard against the drug companies,” Bennett said. “Andrew von Eschenbach, the former head of the FDA, made it very clear that the FDA’s client was the pharmaceutical industry.”
He explained: “If you work at the FDA, and are an underpaid government employee — you maybe have a good heart — but if you decide you want a better paying job, what do you do? You take a job with the pharmaceutical industry. If your resume shows you’ve been leading the charge to change the labels on a bunch of drugs, the only place that resume is going is in the garbage.”
Bennett said the current method for determining the severity and rarity of a side effect related to any given medication is unacceptable. For one, he said, it is very expensive to get a drug approved for a specific use.
“It costs about $800 million to do the appropriate testing. Many drug companies say, ‘Look, we’ve got a small number of labels we can live with — we’ll let the market take care of the rest.’”
How could a drug be around for 20 years and these side effects remain undocumented? This failure, Bennett said, “reveals, not just a chip in the armor — the armor has fallen off. And this is not the only drug like this — I’ve investigated 50 drugs like this.”
Over the past few months, Bennett has met with 10 U.S. Senators and two Congressmen, including Republican Senators Tim Scott and Lindsey Graham of South Carolina and Democratic Congressman James Clyburn, who represents South Carolina’s 6th Congressional District. He has also made numerous media appearances in support of his citizen petition.
But, Bennett stressed, he is not an advocate. He is a scientist.
“Scientists need a seat at the table,” Bennett said. “And so do these patients. They need a voice.”
Bennett said he is fortunate MUSC and USC support him 100 percent and stand by his work. He is also grateful for an NIH research grant to fund that work.
“In my life,” he said, “I always wanted to be in a position to make a difference. I think that’s what we’re doing. We have saved thousands of lives and billions of dollars. That’s a good day’s work.”