Orthomolecular psychiatric therapy is the treatment of mental disease by the provision of the optimum molecular environment for the mind, especially the optimum concentrations of substances normally present in the human body (1). An example is the treatment of phenylketonuric children by use of a diet containing a smaller than normal amount of the amino acid phenylalanine. Phenylketonuria (2) results from a genetic defect that leads to a decreased amount or effectiveness of the enzyme catalyzing the oxidation of phenylalanine to tyrosine. The patients on a normal diet have in their tissues abnormally high concentrations of phenylalanine and some of its reaction products, which, possibly in conjunction with the decreased concentration of tyrosine, cause the mental and physical manifestations of the disease (mental deficiency, severe eczema, and others). A decrease in the amount of phenylalanine ingested results in an approximation to the normal or optimum concentrations and to the alleviation of the manifestations of the disease, both mental and physical.
The functioning of the brain is dependent on its composition and structure; that is, on the molecular environment of the mind. The presence in the brain of molecules of N,N-diethyl-D-lysergamide, mescaline, or some other schizophrenogenic substance is associated with profound psychic effects (3). Cherkin has recently pointed out (4) that in 1799 Humphry Davy described similar subjective reactions to the inhalation of nitrous oxide. The phenomenon of general anesthesia also illustrates the dependence of the mind (consciousness, ephemeral memory) on its molecular environment (5).
The proper functioning of the mind is known to require the presence in the brain of molecules of many different substances. For example, mental disease, usually associated with physical disease, results from a low concentration in the brain of any one of the following vitamins: thiamine (B1), nicotinic acid or nicotinamide (B3), pyridoxine (B6), cyanocobalamin (B12), biotin (H), ascorbic acid (C), and folic acid. There is evidence that mental function and behavior are also affected by changes in the concentration in the brain of any of a number of other substances that are normally present, such as L(+)-glutamic acid, uric acid, and gamma-aminobutyric acid (6).
Optimum Molecular Concentrations
Several arguments may be advanced in support of the thesis that the optimum molecular concentrations of substances normally present in the body may be different from the concentrations provided by the diet and the gene controlled synthetic mechanisms, and, for essential nutrilites (vitamins, essential amino acids, essential fatty acids) different from the minimum daily amounts required for life or the "recommended" (average) daily amounts suggested for good health. Some of these arguments are presented in the following paragraphs.
Evolution and Natural Selection
The process of evolution does not necessarily result in the normal provision of optimum molecular concentrations. Let us me ascorbic acid as an example. Of the mammals that have been studied in this respect, the only species that have lost the power to synthesize ascorbic acid and that accordingly require it in the diet are man, other Primates (rhesus monkey, Formosan long-tail monkey, and ring-tail or brown capuchin monkey), the guinea pig, and an Indian fruit-eating bat (Pteropus medius) (7). Presumably the loss of the gene or genes controlling the synthesis of the enzyme or enzymes involved in the conversion of glucose to ascorbic acid occurred some 20 million years ago in the common ancestor of man and other Primates, and occurred independently for the guinea pig and for one species of bat and one bird, in each case in an environment such that ascorbic acid was provided by the food. For a mutation rate of 1/20,000 per gene generation and for even a very small advantage for the mutant (0.01 percent more progeny) the mutant would replace the earlier genotype within about I million years. The advantage to the mutant of being rid of the ascorbic-acid-synthesis machinery (decrease in cell size and energy requirement, liberation of machinery for other purposes) might well be large, perhaps as much as I percent; a disadvantage nearly as large (less by 0.01 percent) resulting from a less than optimum supply of dietary ascorbic acid would not prevent the replacement of the earlier species by the mutant. Hence, even if the amount of the vitamin provided by the diet available at the time of the mutation were less than the optimum amount, the mutant might still be able to replace its predecessor. Moreover, it is possible that the environment has changed during the last 20 million years in such a way as to provide a decreased amount of the vitamin. Even a serious disadvantage of the changed environment would not lead to a mutation restoring the synthetic mechanism within a period of a few million years, because of the small probability of such mutations, far smaller than of those resulting in loss of function.
Fig. 1. Diagrammatic representation of growth rate or other vital property of an organism as function of the concentration of vital substance in the organism, showing the concentration at which the differential advantage of an increased amount of vital substance is just balanced by the differential disadvantage resulting from an increased amount of machinery for synthesis, and the concentration that gives optimum functioning without consideration of the burden of the machinery for synthesis. Fig. 2 (right). The observed rate of growth of a pyridoxine-requiring Neurospora mutant (Beadle and Tatum, 1941), as function of the concentration of pyridoxine in the medium.

Moreover, the process of natural selection may be expected later on to
lead to the survival of a species or strain that synthesizes somewhat less
than the optimum amount of an autotrophic vital substance rather than of the
species or strain that synthesizes the optimum amount. To synthesize the
optimum amount requires about twice as much biological machinery as to
synthesize half the optimum amount. As suggested in Fig. 1, the evolutionary
disadvantage of synthesizing a less than optimum amount of the vital
substance may be small, and may be outweighed by the advantage of requiring
a smaller amount of biological machinery. Evidence from the study of
microorganisms is discussed in the following paragraphs.
Evidence from Microbiological Genetics
Many mutant microorganisms are known to require, as a supplement to the
medium in which they are grown, a substance that is synthesized by the
corresponding wild-type organism (the normal strain). An example is the
pyridoxine-requiring mutant* of Neurospora sitophila reported by G. W.
Beadle and E. L. Tatum in their first
Neurospora paper, published in 1941 (8). Several species of Neurospora that
have been extensively studied are known to be able to grow satisfactorily on
synthetic media containing inorganic salts, an inorganic source of nitrogen,
such as ammonium nitrate, a suitable source of carbon, such as sucrose, and
the vitamin biotin. All other substances required by the organism are
synthesized by it. Beadle and Tatum found that exposure to x-radiation
produces mutant strains such that one substance must be added to the minimum
medium in order to permit the growth at a rate approximating that of the
normal strain. Their pyridoxine-requiring mutant was found to grow on the
standard medium at a rate only 9 percent of that of the normal strain. When
pyridoxine (vitamin B6) is added to the medium, the rate of growth of this
strain at first increases nearly linearly with the concentration of the
added pyridoxine. and then increases less rapidly, as shown in Fig. 2 (9).
The growth rate of the normal strain without added pyridoxine is equal to
that of the mutant with about 10 micrograms of the growth substance per
liter in the medium. At a concentration about four times this value (40
micrograms per liter) the growth rate of the mutant strain reaches a value 7
percent greater than that of the normal strain without added pyridoxine.
The point of maximum curvature of the curve in Fig. 2, at about 3.2
micrograms of pyridoxine per liter (indicated by a cross), may be reasonably
considered to mark the division between the region of vitamin deficiency (to
the left) and the region of normal vitamin supply (to the right), such as
might permit the mutant to compete with the wild type, which has the growth
rate represented by the filled circle in Fig. 2. The point marked by the
cross might well correspond to an "adequate" or "recommended" amount of the
vitamin, in that the growth rate of the mutant is only 12 percent less than
that of the wild strain, and that the amount of the vitamin would have to be
increased threefold to make up this 12 percent (10).
As shown in Fig. 2, quadrupling the concentration of pyridoxine that gives
the mutant a growth rate equal to that of the wild type causes a further
increase in growth rate by nearly 10 percent. The growth rates of the mutant
and the wild type at very large concentrations of the vitamin have not been
measured, so far as I know, and the optimum concentration is not known. From
the work of Beadle and Tatum the optimum concentration may be taken to be
greater than 40 micrograms per liter; that is, more than ten times the
"adequate" concentration for the mutant and more than four times the
concentration equivalent to the synthesizing capability of the wild type.
The growth rate of the mutant at the optimum concentration is more than 22
percent greater than that at the "adequate" concentration and more than 9
percent greater than that of the normal strain.
Similar results have been reported for other mutants of Neurospora. The
values found by Tatum and Beadle (11) for a p-aminobenzoic-acid-requiring
mutant of Neurospora crassa as a function of the concentration of
p-aminobenzoic acid added to the standard medium are shown in Fig. 3. The
growth-rate curve is similar in shape to that for the pyridoxine-requiring
mutant. The value of the growth rate for the normal strain of Neurospora
crassa with no added p-aminobenzoic acid is equal to that for the mutant at
a concentration of added p-aminobenzoic acid of about 15 micrograms per
liter. A value about 4 percent greater is found for the normal strain at 40
micrograms per liter and for the mutant strain at 80 micrograms per liter,
as indicated in Fig. 3.
Fig. 3 (left). The observed rate of growth of a
p-aminobenzoic-acid-requiring Neurospora mutant (Tatum and Beadle, 1942), as
function of concentration of the growth substance in the medium. Fig. 4
(right). Observed rate of growth of a paraminobenzoic acid-requiring
Neurospora mutant as function of the logarithm of the concentration of
p-aminobenzoic acid.

It is customary to plot values of the growth rate against the logarithm
of the concentration of the growth substance, as shown in Fig. 4. The amount
of increase accompanying a doubling in the concentration of the growth
substance is a maximum at 1.25 to 2.5 micrograms per liter, and decreases
thereafter to about half the value for each successive doubling.
From these two examples we see that there may be a significant increase in
rate of growth of the normal strain through addition of some of the growth
substance that it synthesizes to the medium in which it is grown; that is,
that the amount of the growth substance that is synthesized by the normal
strain is not the optimum amount, but is somewhat less- approximately 7
percent less in the case of pyridoxine (with the normal strain of Neurospora
sitophila) and 4 percent less for p-aminobenzoic acid (with the normal
strain of Neurospora crassa). Many other examples are known of
microorganisms that grow more abundantly in a medium containing vitamins,
amino acids, or other substances that they are able to synthesize than on a
minimum medium.
Evidence supporting the above arguments has been presented recently by
Zamenhof and Eichhorn (11a) in a paper entitled "Study of microbial
evolution through loss of biosynthetic functions: Establishment of
'defective' mutants." These authors carried out experiments involving
competitive growth in a chemostat of an auxotrophic mutant (a mutant
requiring a nutrilite) and a prototrophic parent in a medium of constant
composition containing the nutrilite. They found that the "defective" mutant
has a selective advantage over the prototrophic parental strain under these
conditions. For example, an indole-requiring mutant of Bacillus subtilis was
found to show a strong selective advantage over the prototrophic back-mutant
when the two were grown together in a medium containing tryptophan; the
relative number of cells of the latter decreased 10(6)-fold in 54
generations. They also found that greater advantage to the auxotroph
accompanies a greater number of biosynthetic steps that have been dispensed
with (earlier block in a series of reactions), with the final metabolite
available. They point out that a mutant with a gene deletion would be at a
distinct selective advantage over a point mutant, in that not only the
synthesis of the metabolite, but also that of the structural gene, the
messenger RNA, and perhaps the inactive enzyme itself would be dispensed
with, and that accordingly the mutant with a deletion would replace the
point mutant in competition. They mention evidence that some of the
"defective" strains occurring in nature have lost one or more of their
structural genes by deletions, rather than by point mutations.
Molecular Concentrations and Rate of Reaction
Most of the chemical reactions that take place in living organisms are
catalyzed by enzymes. The mechanisms of enzyme-catalyzed reactions in
general involve (i) the formation of a complex between the enzyme and a
substrate molecule and (ii) the decomposition of this complex to form the
enzyme and the products of the reaction. The rate determining step is
usually the decomposition of the complex to form the products, or, more
precisely, the transition through an intermediate state of the complex,
characterized by activation energy less than for the uncatalyzed reaction,
to a complex of the enzyme and the products of reaction, with a rapid
dissociation. Under conditions such that the concentration of the complex
corresponds to equilibrium with the enzyme and the substrate, the rate of
the reaction is given by the following equation [the Michaelis-Menten
equation (12)]:
![]()
In this equation [S] is the concentration of the substrate, E is the
total concentration of enzyme (present both as free enzyme and enzyme
complex), K is the equilibrium constant for formation of the enzyme complex
ES, and k is the reaction-rate constant for decomposition of the complex to
form the enzyme and reaction products. This equation corresponds to the case
in which there are no enzyme inhibitors present.
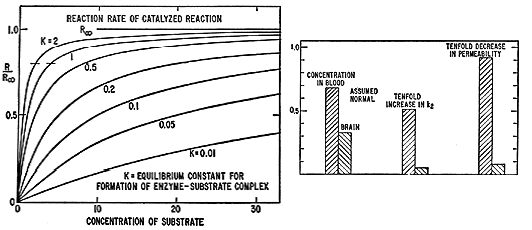
Values of the reaction rate calculated from this equation for different
values of K are shown in Fig. S. The curves are similar in shape to those of
Figs. 2 and 3. At concentrations much smaller than K (-1) the reaction rate
is proportional to the concentration of substrate. At larger concentrations,
as the amount of enzyme complex becomes comparable to the amount of free
enzyme, the reaction rate changes from the linear dependence. At substrate
concentration equal to K (-1) the slope of the curve is one-quarter of the
initial slope, and the value is one-half of the value corresponding to
saturation of the enzyme by the substrate.
The similarity of the curves of Figs. 2 and 3 to appropriate curves in Fig.
5 suggests that the growth substance may be involved in an enzyme-catalyzed
reaction in which it serves as the substrate. The normal strain of the
organism manufactures an amount of the substrate such as to permit the
reaction to take place at what may be considered a normal rate, 90 or 95
percent of the maximum rate, which corresponds to saturation of the enzyme.
As described above, the gain in reaction rate associated with the
manufacture of a larger amount of the substrate, with a corresponding
advantage to the organism, might be balanced by the disadvantage to the
organism associated with the upkeep of the larger amount of machinery
required to manufacture the increased amount of substrate. An increase in
rate of this reaction could also be achieved by an increase in the amount of
the enzyme synthesized by the organism. Here, again, the advantage to the
organism resulting from this increase may be overcome by the disadvantage
associated with the increase in the amount of machinery required for the
increased synthesis. During the process of evolution there has presumably
been selection of genes determining the concentrations of the enzymes
catalyzing successive reactions such as to achieve an approximation to the
optimum reaction rate with the smallest amount of disadvantage to the
organism.
The rate of an enzyme-catalyzed reaction is approximately proportional to
the concentration of the reactant, until concentrations that largely
saturate the enzyme are reached. The saturating concentration is larger for
a defective enzyme with decreased combining power for the substrate than for
the normal enzyme. For such a defective enzyme the catalyzed reaction could
be made to take place at or near its normal rate by an increase in the
substrate concentration, as indicated in Fig. 5. The short horizontal lines
intersecting the curves indicate what may be called the "normal" reaction
rate, 80 percent of the maximum. For K = 2 the "normal" rate is achieved at
substrate concentration [S] = 2. At this substrate concentration the
reaction rate is only 29 percent of the maximum and 35 percent of "normal"
for a mutated enzyme with K = 0.2; it could be raised to the "normal' value
by a tenfold increase in the substrate concentration, to [S] = 20.
Similarly, the still greater disadvantage of low reaction rate for a mutated
enzyme with K only 0.01 could be overcome by a 200-fold increase in
substrate concentration, to [S] = 400, This mechanism of action of gene
mutation is only one of several that lead to disadvantageous manifestations
that could be overcome by an increase, perhaps a great increase, in the
concentration of a vital substance in the body. These considerations
obviously suggest a rationale for megavitamin therapy.
Molecular Concentrations and Mental Disease
The functioning of the brain and nervous tissue is more sensitively
dependent on the rate of chemical reactions than the functioning of other
organs and tissues. I believe that mental disease is for the most part mused
by abnormal reaction rates, as determined by genetic constitution and diet,
and by abnormal molecular concentrations of essential substances. The
operation of chance in the selection for the child of half of the complement
of genes of the father and mother leads to bad as well as to good genotypes,
and the selection of foods (and drugs) in a world that is undergoing rapid
scientific and technological change may often be far from the best.
Significant improvement in the mental health of many persons might be
achieved by the provision of the optimum molecular concentrations of
substances normally present in the human body. Among these substances, the
essential nutrilites may be the most worthy of extensive research and more
thorough clinical trial than they have yet received. One important example
of an essential nutrilite that is required for mental health is vitamin B12,
cyanocobalamin. A deficiency of this vitamin, whatever its cause (pernicious
anemia; infestation with the fish tapeworm Diphyllobothrium, whose high
requirement for the vitamin results in deprivation for the host; excessive
bacterial flora, also with a high vitamin requirement, as may develop in
intestinal blind loops), leads to mental illness, often even more pronounced
than the physical consequences. The mental illness associated with
pernicious anemia [a genetic defect leading to deficiency of the intrinsic
factor (a mucoprotein) in the gastric juice and the consequent decreased
transport of cyanocobalamin into the blood] often is observed for several
years in patients with this disease before any of the physical
manifestations of the disease appear (13). A pathologically low
concentration of cyanocobalamin in the serum of the blood has been reported
to occur for a much larger fraction of patients with mental illness than for
the general population. Edwin, Holten, Norum, Schrumpf, and Skaug (14)
determined the amount of B12 in the serum of every patient over 30 years old
admitted to a mental hospital in Norway during a period of I year. Of the
396 patients, 5.8 percent (23) had a pathologically low concentration, less
than 101 picograms per millilitre, and the concentration in 9.6 percent (38)
was subnormal (101 to 150 picograms per millilitre). The normal
concentration is 150 to 1300 picograms per millilitre. The incidence of
pathologically low and subnormal levels of B1, in the serums of these
patients, 15.4 percent, is far greater than that in the general population,
about 0.5 percent (estimated from the reported frequency of pernicious
anemia in the area, 9.3 per 100,000 persons per year). Other investigators
(15) have also reported a higher incidence Of low B12 concentrations in the
serums of mental patients than in the population as a whole, and have
suggested that B12 deficiency, whatever its origin, may lead to mental
illness.
Nicotinic acid (niacin), when its use was introduced, cured hundreds of
thousands of pellagra patients of their psychoses, as well as of the
physical manifestations of their disease. For this purpose only small doses
are required; the recommended daily allowance (National Research Council) is
12 milligrams per day (for a 70-kilogram male). In 1939 Cleckley,
Sydenstricker, and Geeslin (16) reported the Successful treatment of 19
patients and in 1941 Sydenstricker and Cleckley (17) reported similarly
successful treatment of 29 patients with severe psychiatric symptoms by use
of moderately large doses of nicotinic acid (0.3 to 1.5 grams per day). None
of these patients had physical symptoms of pellagra or any other
avitaminosis. More recently many other investigators have reported on the
use of nicotinic acid and nicotinamide for the treatment of mental disease.
Outstanding among them are Hoffer and Osmond, who since 1952 have advocated
and used nicotinic acid in large doses. in addition to the conventional
therapy, for the treatment of schizophrenia (18-20). The dosage recommended
by Hoffer is 3 to 18 grams per day, as determined by the response of the
patient, of either nicotinic acid or nicotinamide, together with 3 grams per
day of ascorbic acid. Nicotinic acid and nicotinamide are nontoxic [the
lethal dose, 50 percent effective (LD50), is not known for humans, but
probably it is over 200 grams; the LD,50 for rats is 7.0 grams per kilogram
for nicotinic acid, and 1.7 grams per kilogram for nicotinamide], and their
side effects, even in continued massive doses, seem not to be commonly
serious. Among the advantages of nicotinic acid, summarized by Osmond and
Hoffer (19), are the following: it is safe, cheap, and easy to administer,
and it is a well-known substance that can be taken for years on end, if
necessary, with only small probability of incidence of unfavourable side
effects.
Another vitamin that has been used to some extent in the treatment of mental
disease is ascorbic acid, vitamin C. A sometimes-recommended daily intake of
ascorbic acid is 75 milligrams for healthy adults. Some investigators have
estimated that the optimum intake is much larger (21): perhaps 3 to 15 grams
per day, according to Stone (22). Williams and Deason (23) have emphasized
the variability of individual members of a species of animals; they have
reported their observation of a 20-fold range of required intake of ascorbic
acid by guinea pigs, and have suggested that human beings, who are less
homogeneous, have a larger range.
Mental symptoms (depression) accompany the physical symptoms of vitamin-C
deficiency disease (scurvy). In 1957 Akerfeldt (24) reported that the serum
of schizophrenics had been found to have greater power of oxidizing N,N
dimethyl-p-phenylenediamine than that of other persons. Several
investigators then reported that this difference is due to a smaller
concentration of ascorbic acid in the serum of schizophrenics than of other
persons. This difference has been attributed to the poor diet and increased
tendency to chronic infectious disease of the patients (25), and has also
been interpreted as showing an increased rate of metabolism of ascorbic acid
by the patients (26). It is my opinion, from the study of the literature,
that many schizophrenics have an increased metabolism of ascorbic acid,
presumably genetic in origin, and that the ingestion of massive amounts of
ascorbic acid has some value in treating mental disease.
Other vitamins (thiamine, pyridoxine, folic acid) and other substances [zinc
ion, magnesium ion, uric acid, tryptophan, L(+)-glutamic acid, and others]
influence the functioning of the brain. I shall review work on L(+)-glutamic
acid as a further example. L(+)-Glutamic acid is an amino acid that is
present at rather high concentration in brain and nerve tissue and plays an
essential role in the functioning of these tissues (27). It is normally
ingested (in protein) in amounts of 5 to 10 grams per day. It is not toxic;
large doses may cause increased motor activity and nausea. In 1944 Price,
Waelsch, and Putnam (28) reported favourable results for glutamic acid
therapy of convulsive disorders [benefit to one Out of three or four
patients with petit mal epilepsy (29)]. Zimmerman and Ross then reported an
increase in maze-running learning ability of white rats given extra amounts
of glutamic acid (30). Zimmerman and many other investigators then studied
the effects of glutamic acid on the intelligence and behavior of persons
with different degrees and kinds of mental retardation. L(+)-Glutamic is
apparently more effective than its sodium or potassium salts. The effective
dosage is usually between 10 and 20 grams per day (given in three doses with
meals), and is adjusted to the patient as the amount somewhat less than that
required to cause hyperactivity; improvement in personality and increase in
intelligence (by 5 to 20 I.Q. points) have been reported for many patients
with mild or moderate mental deficiency by several investigators (31).
Fig. 5 (left). Curves showing calculated reaction rate RIR. of catalyzed reaction as function of the concentration of the substrate, for different values of the equilibrium constant K for formation of the enzyme-substrate complex. Fig. 6 (right). Values of the concentration of a vital substance in the blood and in the cerebrospinal fluid for three different assumed sets of value of bloodbrain barrier permeability and rate of destruction in the cerebrospinal fluid.
Localized Cerebral Deficiency Diseases
The observation that the psychosis associated with pernicious anemia may
manifest itself in a patient for several years before the other
manifestations of this disease become noticeable has a reasonable
explanation: the functioning of the brain and nervous tissue is probably
more sensitively dependent on molecular composition than is that of other
organs and tissues. The observed high incidence of cyanocobalamin deficiency
in patients admitted to a mental hospital, mentioned above, suggests that
mental disease may rather often be the result of this deficiency, and
further suggests that other deficiencies in vital substances may be wholly
or partly responsible for many cases of mental illness.
The foregoing arguments suggest the possibility that under certain
circumstances a deficiency disease may be localized in the human body in
such a way that only some of the manifestations usually associated with the
disease are present. Let us consider, for example, an enzyme or other vital
substance that is normally metabolized by the catalytic action of an enzyme
normally present in the tissues and organs of the body. In a person of
unusual genotype there might be an especially great concentration of this
enzyme in one body organ, with essentially the normal amount in other
organs. Through the action of this enzyme in especially great concentration
the steady-state concentration of the vital substance in that organ might be
decreased to a level much lower than that required for normal function.
Under these circumstances there would be present a deficiency disease
restricted to that organ.
An especially important case is that of the brain. We may, as a rough model
of the human body, consider two reservoirs of fluid, the blood and lymph,
with volume VI, and cerebrospinal fluid, the extracellular fluid of the
brain and spinal column, with volume V2. We assume that a vital substance is
destroyed in each of these reservoirs at a characteristic rate,
corresponding to the rate constants k1 and k2, that it diffuses across the
blood-brain barrier at a rate determined by the product of the permeability
and area of the barrier and the difference c2 - c1 of the concentrations in
the two reservoirs, and that it is introduced from the gastrointestinal
tract into the first reservoir at a constant rate. The steadystate
concentrations are then in the ratio
c1/c2 = 1 + (K2V2/PA)
where PA is the product of permeability and the area of the blood-brain
barrier. The steady state corresponds to the following system:

From this equation it is seen, as shown also in Fig. 6, that for small
values of k2V2/PA the difference in steady-state concentrations in the
cerebrospinal fluid and the blood is small, but that through either decrease
in permeability of the barrier or increase in the metabolic rate constant k2
the steady-state concentration in the brain becomes much less than that in
the blood.
This simple argument leads us to the possibility of a localized cerebral
avitaminosis or other localized cerebral deficiency disease. There is the
possibility that some human beings have a sort of cerebral scurvy, without
any of the other manifestations, or a sort of cerebral pellagra, or cerebral
pernicious anemia. It was pointed out by Zuckerkandl and Pauling (32) that
every vitamin, every essential amino acid, every other essential nutrilite
represents a molecular disease (33) which our distant ancestors learned to
control, when it began to afflict them, by selecting a therapeutic diet, and
which has continued to be kept under control in this way. The localized
deficiency diseases described above are also molecular diseases, compound
molecular diseases, involving not only the original lesion, the loss of the
ability to synthesize the vital substance, but also another lesion, one that
causes a decreased rate of transfer across a membrane, such as the
bloodbrain barrier (34), to the affected organ, or an increased rate of
destruction of the vital substance in the organ, or wine other perturbing
reaction,
It has been suggested by Huxley, Mayr, Osmond, and Hoffer (35), partially on
the basis of the observations of Böök (36) and Slater (37) on the incidence
of schizophrenia in relatives of schizophrenics, that schizophrenia is
caused by a dominant gene with incomplete penetrance. They suggested that
the penetrance, about 25 percent, may in some cases be determined by other
genes and in some cases by the environment. I suggest that the other genes
may in most cases be those that regulate the metabolism of vital substances,
such as ascorbic acid, nicotinic acid or nicotinamide, pyridoxine,
cyanocobalamin, and other substances mentioned above, The reported success
in treating schizophrenia and other mental illnesses by use of massive doses
of wine of these vitamins may be the result of successful treatment of a
localized cerebral deficiency disease involving the vital substances,
leading to a decreased penetrance of the gene for schizophrenia. There is a
possibility that the so-called gene for schizophrenia is itself a gene
affecting the metabolism of one or another of these vital substances, or
even of several vital substances, causing a multiple cerebral deficiency.
I suggest that the orthomolecular treatment of mental disease, to be
successful, should involve the thorough study of and attention to the
individual, such as is customary in psychotherapy but less customary in
conventional chemotherapy. In the course of time it should be possible to
develop a method of diagnosis (measurement of concentrations of vital
substances) that could be used as the basis for determining the optimum
molecular concentrations of vital substances for the individual patient and
for indicating the appropriate therapeutic measures to be taken. My
co-workers and I are carrying on some experimental studies suggested by the
foregoing considerations, and hope to be able before long to communicate
some of our results.
Summary
The functioning of the brain is affected by the molecular concentrations
of many substances that are normally present in the brain. The optimum
concentrations of these substances for a person may differ greatly from the
concentrations provided by his normal diet and genetic machinery.
Biochemical and genetic arguments support the idea that orthomolecular
therapy, the provision for the individual person of the optimum
concentrations of important normal constituents of the brain, may be the
preferred treatment for many mentally ill patients. Mental symptoms of
avitaminosis sometimes are observed long before any physical symptoms
appear. It is likely that the brain is more sensitive to changes in
concentration of vital substances than are other organs and tissues.
Moreover, there is the possibility that for some persons the cerebrospinal
concentration of a vital substance may be grossly low at the same time that
the concentration in the blood and lymph is essentially normal. A
physiological abnormality such as decreased permeability of the bloodbrain
barrier for the vital substance or increased rate of metabolism of the
substance in the brain may lead to a cerebral deficiency and to a mental
disease. Diseases of this sort may be called localized cerebral deficiency
diseases. It is suggested that the genes responsible for abnormalities
(deficiencies) in the concentration of vital substances in the brain may be
responsible for increased penetrance of the postulated gene for
schizophrenia, and that the so-called gene for schizophrenia may itself -be
a gene that leads to a localized cerebral deficiency in one or more vital
substances.
References and Notes
1.I might have described this therapy as the provision of the optimum
molecular composition of the brat.. Th. brain provides the molecular
environment of the mind. I use the word mind as a convenient synonym for the
functioning of the brain, The word orthomolecular may be criticized as a
Greek-Latin hybrid. I have suit, however, found any other word that
expresses a well the idea of the right molecules in the right amounts
2.A. Felling, Nord. Med. Tidsk,. 9, 1054 (1934). Z. Physiol. Chem. 277, 169
(1934).
3.See, for example, D. W. Woolley, The Biochemical Bases of Psychoses
(Wiley. New York, 1962).
4.A. Cherkin, Science 155, 266 (1967).
5. L. Pauling Ibid. 134, 15 (1961); S. Miller, Proc Not. Acad. Set. U.S. 47,
1515 (1961).
6.The literature Is so extensive that I refrain from giving references, here
7. For references see, 1. Stone, Amer. J. Phys. Anthropol. 23, 93 (1965).
The only other vertebrate known to require exogenous ascorbic acid is the
red-vented bulbul Pycnonotus rates.
8. G. W. Beadle and E. L. Tatum, Proc. Nat. Acad. Sci. U.S. 27, 499 (1941).
9. The Points in Fig. 2 =present my measurement of the stripes of the growth
curves shown in fig. 1 of reference (8)- They agree closely with the points
of fig. 2 of reference (8) except for one Point, that for 1.2 µg/liter,
which may have been misplotted.
10.The reported growth rate for the normal strain in a medium with 40 as of
added pyridoxine, Per liter is 3 percent greater than that for the basic
medium, as shown by the slopes of the lines in reference (8), fig. 1.
11. E. L. Tatum ad G. W. Beadle, Proc. Nat Acad. Set. U.S, 28, 234 (1942).
11a. S. Zamenhof and H. H. Eichhorn, Nature 216, 465 (1967).
12.L. Michaelis and M. Menten, Biochem. Z. 49, 333 (1913).
13.A. D. M. Smith, Brit. Med. J. 11, 1840 (1950).
14. R. Edwin, K. Holten K.R. Norum. A. Schrumf, 0.E. Skaug, Act. Med. Scand.
171: 689 (1965).
15. T. Ran.. 0. J. Rafaelson P. Rødbro, Laurel 1966-II. 965 . (1966). report
serum Br concentration below 150 pg/ml in 13 of I" consecutive patients
admitted to a Copenhagen psychiatric clinic; J. G. Henderson, R. W.
Strachan. J. S. Beck, A. A. Dawson, M. Daniel, ibid., p. 809. report that
nine of 1012 unselected Psychiatric patients in a region in Scotland were
found to have B12 deficiency, in addition to five pernicious anemia patients
in the group.
16. H. M. Cleckley, V. P. Sydenstricker, L. F. Geeslin, J. Amer. Med. Ass.
112, 2107 (1939)
17. V. P. Sydenstricker and 14. M. Cleckley, Amer. J. Psychiat. 99 83
(1941). References am given in this paper to some earlier work on nicotinic
acid therapy.
18. A. Hoffer, H. Osmond, M. J. Callbeck, I Kahan, J Clin Exp Psychopathol
18, 131 (1957); A. Hoffer, Niacin Therapy I. Psychiat, (Thomas, Springfield,
Ill., 1962).
19. H. Osmond & A. Hoffer, Lancet 1962-II, 316 (1962); review of a 9-year,
study.
20. A. Hoffer & H. Osmond, Acid Psychiat. Scand. 40, 171 (1964); A- Hoffer,
Int. J. Neuropsychiat 2, 234 (1966).
21.For example, E. D. Kyhos, E. I. Sevringhaus, D. R. Hagendorn, Arch. Int.
Med. 75, 407 (1945), found that for wine subjects 1.5 to 2.8 grams. Per day
as- needed for saturation.
22. I. Stone, Perspect. Biol. Med. 10, 135 (1967); Act. Genet Med Gemell.
15, 345 (1966).
23. R. J. Williams and G. Deason, Proc. Nat. Acad. Sci. U.S. 37, 1638
(1967).
24. S. A. Akerfeldt, Science 125, 117 (1957).
25. J. D. Benjamin, Psychosom. Med. 20, 427 (1958); S. S. Kety, Science 129,
1528, 1590 (1959).
26. A. Hoffer and H. Osmond, The chemical Basis of Clinical Psychiatry,
(Thomas, Springfield, Ill., 1960), p. 232; M. H. Briggs, New. Zealand Med.
J. 61, 229 (1962),
27. H. Weil-Malherbe Mechem J. 30, 665 (1936).
29. J. G. price, H. Waelsch, T. J. Parallel, J. Amer. Med. Ass. 122 (1944).
29. H. Waelsch Amer. J. Ment.. Defic. 52, 305,(1948)
30.F. T. Zimmermam and S. Ross, Arch. Neurol. Psychiat. 51, 446 (1944).
31. A recent survey of the role of glutamic acid in cognitive behaviors has
been published by W. Vogel, D. M. Broverman, J. 0. Draguns, E, L. Klaiber,
Psychol. Bull. 65, 367 (1966)- Many references, to earlier work are given in
this Paper.
32. E. Zuckerkandl and L. Panting, in Horizon. in Biochemistry, M. Kasha and
R. Pullman, Eds. (Academic Press, New York, 1962), P. 189
33. L. Painting, H. A. Itano, S. 1. Singer, I C. Wells, Science 110, 543
(1949).
34. It has been suggested by B. Melander and S. Martens, Dis. Nerv. Syst.
19, 478 (1959); Acta Psychiat. Neurol. Scand. 34, 344 (1959). and by A.
Hoffer and H. Osmond, Int. J. Neuropsychiat. 2, 1 (1966), that the effects
of taraxein. [R. G. Heath. S. Martens, B. E. Leach, M. Cohen, C. A- Feigley,
Amer. J. Psychiat. 114, 917 (1958)] may result from changing the
permeability of the blood-brain barrier.
35. J. Huxley, E. Man. H. Osmond, A. Hoffer, Nature 204, 220 (1964),
36. J. A. Böök, Arm Genet. Stairs. Med. 4 (1) (1953); Proc. Int. Congr.
Genet. 10th 1, 81 (1958).
37.I. E. Slater. Acta Genet. Statist. Mail. 9, 50 (1958).