In September 2000, two instances of life-threatening hepatotoxicity were reported in health-care workers taking nevirapine (NVP) for postexposure prophylaxis (PEP) after occupational human immunodeficiency virus (HIV) exposure*. In one case, a 43-year-old female health-care worker required liver transplantation after developing fulminant hepatitis and end-stage hepatic failure while taking NVP, zidovudine, and lamivudine as PEP following a needlestick injury (1). In the second case, a 38-year-old male physician was hospitalized with life-threatening fulminant hepatitis while taking NVP, zidovudine, and lamivudine as PEP following a mucous membrane exposure. To characterize NVP-associated PEP toxicity, CDC and the Food and Drug Administration (FDA) reviewed MedWatch reports of serious adverse events in persons taking NVP for PEP received by FDA (Figure 1). This report summarizes the results of that analysis and indicates that healthy persons taking abbreviated 4-week NVP regimens for PEP are at risk for serious adverse events. Clinicians should use recommended PEP guidelines and dosing instructions to reduce the risk for serious adverse events.
MedWatch is a voluntary reporting system for adverse events and problems with drugs, medical devices, biologics, and special nutritional products. For this analysis, a serious adverse event was defined as any event that was life-threatening, permanently disabling, required or prolonged hospitalization, required intervention to prevent permanent impairment or damage, or any other event that required medical attention.
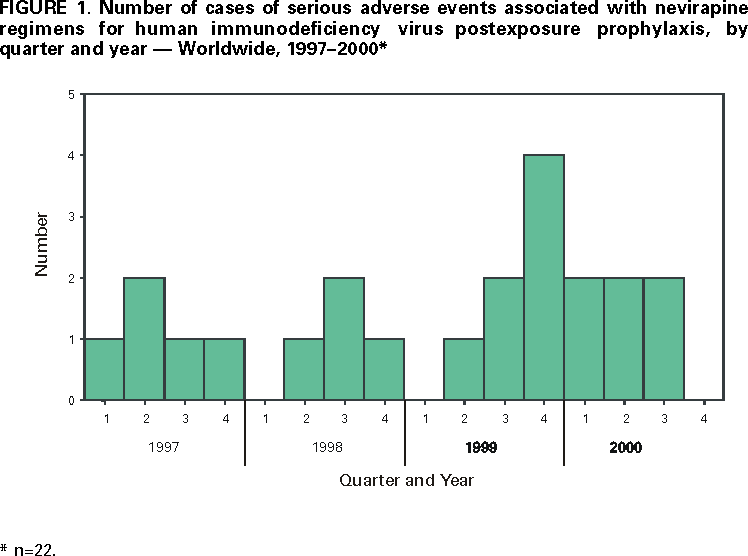
Including the two case reports of fulminant hepatitis, FDA received reports of 22 cases of serious adverse events related to NVP taken for PEP from March 1997 through September 2000. These 22 events included hepatotoxicity (12), skin reaction (14), and rhabdomyolysis (one); four cases involved both hepatotoxicity and skin reaction, and one case involved both rhabdomyolysis and skin reaction. The median age of affected persons was 36.5 years (range: 12--50 years; age was not reported for four cases); 12 were female, and 12 occurred in the United States. Reasons for administration of PEP were occupational needlestick or other sharps injury (12), other occupational exposure (four), sexual exposure (three), nonoccupational (pediatric) needlestick injury (one), other nonoccupational exposure (one), and unknown (one).
Nine persons took a maximum NVP dose of 200 mg per day, and 12 persons took a maximum dose of 200 mg twice per day (the dose of NVP was not recorded for one person). Among the 12 persons taking a maximum dose of 200 mg twice daily, six were first given a lead-in dose of 200 mg per day for 3--14 days. Concomitant antiretroviral agents used with NVP for PEP included zidovudine and lamivudine (10); stavudine and lamivudine (three); zidovudine and didanosine (two); stavudine and didanosine (one); stavudine and indinavir (one); didanosine and indinavir (one); stavudine, didanosine, and ritonavir (one); lamivudine, didanosine, and nelfinavir (one); stavudine, lamivudine, nelfinavir, and saquinavir (one); and none (one). Among the 12 persons with hepatotoxic reactions, one developed liver failure (requiring liver transplantation), seven had clinical hepatitis (e.g., jaundice, fever, nausea, vomiting, abdominal pain, and/or hepatomegaly), and four had elevations in serum liver enzymes (i.e., alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) without reports of clinical hepatitis.
Baseline liver function tests were reported for six patients and were within normal limits. Abnormal liver function tests were reported during PEP for 10 patients; median peak ALT was 215 U/L (range: 182--2790 U/L; normal: 10--34 U/L), median peak AST was 375 U/L (range: 96--2370 U/L; normal: 10--34 U/L), and median peak total bilirubin was 7.5 mg/dL (range: 2.0--33.7 mg/dL; normal: 0.2--1.0 mg/dL). The median time from initiation of NVP use to first abnormal liver function tests was 21 days (range: 13--36 days). In six cases, hepatitis A, B, and C serologies were reported; all were negative. Eleven persons reported symptoms, including fever, malaise, and abdominal pain. The median onset of these symptoms was 14 days after beginning NVP for PEP (range: 3--36 days). The 14 reports of skin rash included one documented and two possible cases of Stevens-Johnson syndrome. The median onset of rash occurred 9 days after beginning PEP (range: 6--36 days).
Reported by: D Boxwell, Pharm D, Office of Postmarketing Drug Risk Assessment; H Haverkos, MD, S Kukich, MD, K Struble, Pharm D, H Jolson, MD, Div of Anti-Viral Drug Products, Center for Drug Evaluation and Research, Food and Drug Administration. Prevention and Evaluation Br, Div of Healthcare Quality Promotion [proposed], National Center for Infectious Diseases, CDC.
Severe, life-threatening, and fatal cases of hepatotoxicity and skin reactions have occurred among HIV-infected patients treated with NVP (2,3) and are described in a box warning on the NVP label (Viramune™ [package insert]†, Boehringer Ingelheim/Roxane Laboratories, Inc., Ridgefield, Connecticut, 1998). This report suggests that persons taking NVP regimens for PEP after HIV exposures also are at risk for serious adverse events.
In 1996, the U.S. Public Health Service (PHS) first recommended PEP after certain occupational exposures to HIV (4). These recommendations, updated in 1998 (5), are being revised to include other antiretroviral agents that have been approved by FDA for use in HIV-infected persons. NVP is not recommended for basic or expanded PEP regimens. However, data on the safe and effective use of single-dose NVP to prevent perinatal HIV transmission (6,7) and a theoretical advantage of more rapid activity (i.e., NVP does not require phosphorylation for activation) have prompted clinicians to include NVP in PEP regimens following HIV exposures. In the HIV PEP registry, which collected data on occupational HIV PEP use from October 1995 through March 1999, six cases of serious adverse events related to PEP were reported among 492 registered participants; a severe skin reaction occurred in one of 11 health-care workers taking a regimen that included NVP (8).
Because most occupational HIV exposures do not result in transmission of HIV (9), clinicians considering prescribing PEP for exposed persons must balance the risk for HIV transmission represented by the exposure and the exposure source against the potential toxicity of the specific agent(s) used (4). In many circumstances, the risks associated with NVP as part of a PEP regimen outweigh the anticipated benefits. When PEP is prescribed, the manufacturer's package insert should be consulted for dosing instructions, possible side effects, and potential drug interactions.
The findings in this report are subject to at least three limitations. First, MedWatch is a voluntary, passive reporting system, and it is unlikely that all serious adverse events in persons taking NVP for PEP have been reported. Second, data about administration of a lead-in dose and results of baseline liver function tests and hepatitis serologies were not included in all reports. In six cases, the initial dose of NVP was 200 mg twice daily without the recommended 2-week dose escalation, which may have increased the likelihood of adverse events (10). Third, available denominator data about the use of NVP for PEP were insufficient to calculate accurate rates of adverse events.
The findings in this report do not apply to NVP use in other settings. Single-dose NVP is one of the regimens recommended by PHS for prevention of perinatal HIV transmission (7). No serious toxicity has been reported among mother-infant pairs using this regimen. Combination antiretroviral regimens containing NVP may be used in HIV- infected persons after weighing the risks and benefits and monitoring adverse reactions.
Health-care providers and the public can assist in monitoring the safety of antiretrovirals and other agents by reporting adverse reactions to the FDA MedWatch program: telephone, (800) 332-1088, fax, (800) 332-0178, World-Wide Web, http://www.FDA.gov/medwatch, or mail, MedWatch, HF-2, FDA, 5600 Fishers Lane, Rockville, MD 20857.
* Information included in this report does not represent Food and Drug Administration approval or approved labeling for the particular product or indications in question.
† Use of trade names and commercial sources is for identification only and does not constitute endorsement by CDC or the U.S. Department of Health and Human Services.
All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
Page converted: 1/4/2001